
Most of his forecasts proved to be correct. He also predicted some properties of unidentified elements that were expected to fill gaps within the table. Russian chemist Dmitri Mendeleev was the first to publish a recognizable periodic table in 1869, developed mainly to illustrate periodic trends of the then-known elements. The organization of the periodic table can be used to derive relationships between the various element properties, but also the predicted chemical properties and behaviors of undiscovered or newly synthesized elements. Also displayed are four simple rectangular areas or blocks associated with the filling of different atomic orbitals. Six groups have accepted names as well as assigned numbers: for example, group 17 elements are the halogens and group 18 are the noble gases. Table rows are commonly called periods and columns are called groups. Generally, within one row (period) the elements are metals to the left, and non-metals to the right, with the elements having similar chemical behaviors placed in the same column.
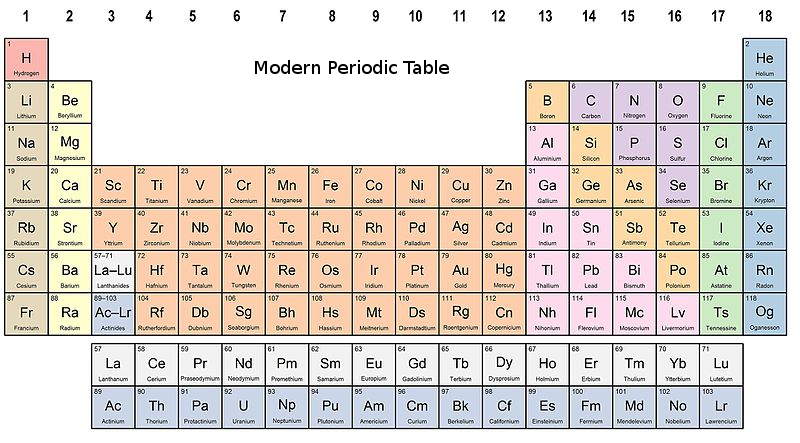
The periodic table is a tabular arrangement of the chemical elements, ordered by their atomic number, electron configuration, and recurring chemical properties, whose structure shows periodic trends. There are many more elements still to be discovered.įor more Chemistry notes for Class 11, 11th Science, NCERT solutions Class 11th, CBSE guide, sample papers, latest updates from CBSE Board, enroll with Takshila Learning.Welcome to the course on ''Learn periodic table with it's basic concepts in Chemistry'' Thus, the total number of elements discovered so far is 109.

The modern periodic table of elements consists of 18 vertical columns (groups) and 7 horizontal rows (periods). It is the present form or the modern periodic table of elements.

The long form of the periodic table is built according to the electronic configuration of the elements. ns1 where n refers to the number of outermost shells (principal quantum number). The elements of group IA are alkali metals which have a similar outer electronic configuration, i.e. The repetition of properties of elements results from alike outer electronic configuration after certain regular intervals. In this form of a periodic table, the horizontal rows are called periods and the vertical columns are known as the groups. The present form of a periodic table which is widely used across the globe is the long form of the periodic table. This periodic table is also known as a Long or Extended form of Periodic Table. Many other chemists also arranged different elements in order of increasing atomic numbers. This law is the basis of formulation of the modern periodic table of elements. NCERT & CBSE Class 11 Chemistry MODERN PERIODIC TABLEĬlass 11 chemistry classes: Modern periodic law(given by Mosley) states that there is a periodic repetition of properties of the elements is arranged in order of their increasing atomic numbers.


 0 kommentar(er)
0 kommentar(er)
